Nematophages fungi in the biocontrol of the Meloidogyne exigua Goeldi in Coffea arabica L. var. catimor, in Satipo - Peru
DOI:
https://doi.org/10.51252/raa.v2i2.343Keywords:
control, dose, Paecilomyces, Pochonia, TrichodermaAbstract
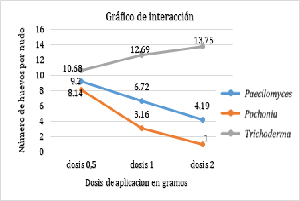
The central jungle of Peru is the most important coffee-growing region at the national level, where there is a high incidence of the nematode that forms galls on the roots of the coffee tree; Meloidogyne exigua Goeldi, without adequate control because chemical control affects health and the environment, in this sense biological control alternatives are sought. The objective was to test the effectiveness of three nematophagous fungi against M. exigua Goeldi using nursery plants. The work was carried out in the laboratory and in the field. The variables evaluated were: number of galls, incidence of nematodes, severity, population of eggs, population of juveniles 2 and population of females. The results show that the 1st application of Pochonia chlamidosporia is statistically superior in the control of nematodes with respect to Paecilomyces lilacinus and Trichoderma harzianum, which occupy the second and third place, respectively. The dose of 2 g/seedling for P. chlamidosporia statistically exceeds the doses of 1.0 and 0.5 g/seedling. When the application rate of Paecilomyces lilacinus and Pochonia chlamydosporia increases, all variables decrease; but when the dose of Trichoderma harzianum increases, the variables remain constant, so there is no control effect.
Downloads
References
Bontempo, A. F., Fernandes, R. H., Lopes, J., Freitas, L. G., & Lopes, E. A. (2014). Pochonia chlamydosporia controls Meloidogyne incognita on carrot. Australasian Plant Pathology, 43(4), 421–424. https://doi.org/10.1007/s13313-014-0283-x
Cleopas C., C., Kwasi S., Y., & Mark D., L. (2017). Biological control of the rootknot nematode, Meloidogyne javanica (Chitwood) using Bacillus isolates, on soybean. Biological Control, 109, 37–41. https://doi.org/10.1016/j.biocontrol.2017.03.009
Dube, B., & Grover C., S. J. (1987). Biological Control of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. Journal of Nematology, 19(2), e227. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2618639/
Esteves, I., Peteira, B., Atkins, S. D., Magan, N., & Kerry, B. (2009). Production of extracellular enzymes by different isolates of Pochonia chlamydosporia. Mycological Research, 113(8), 867–876. https://doi.org/10.1016/J.MYCRES.2009.04.005
Flores-Camacho, R., Manzanilla-López, R. H., Cid del Prado-Vera, I., & Martínez-Garza, Á. (2007). Control de Nacobbus aberrans (Thorne) Thorne y Allen con Pochonia chlamydosporia (Goddard) Gams y Zare. Revista Mexicana de Fitopatología, 25(1), 26–35. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-33092007000100004
Hoedekie, A., Viaene, N., & Van Damme, V. (2005). Long-term efficacy of Pochonia chlamydosporia for management of Meloidogyne javanica in glasshouse crops. Nematology, 7(5), 727–736. https://doi.org/10.1163/156854105775142973
Larriba, E., Jaime, M. D. L. A., Carbonell-Caballero, J., Conesa, A., Dopazo, J., Nislow, C., Martín-Nieto, J., & Lopez-Llorca, L. V. (2014). Sequencing and functional analysis of the genome of a nematode egg-parasitic fungus, Pochonia chlamydosporia. Fungal Genetics and Biology, 65, 69–80. https://doi.org/10.1016/j.fgb.2014.02.002
Luambano, N. D., Manzanilla-López, R. H., Kimenju, J. W., Powers, S. J., Narla, R. D., Wanjohi, W. J., & Kerry, B. R. (2015). Effect of temperature, pH, carbon and nitrogen ratios on the parasitic activity of Pochonia chlamydosporia on Meloidogyne incognita. Biological Control, 80, 23–29. https://doi.org/10.1016/J.BIOCONTROL.2014.09.003
Manzanilla-López, R. H., Esteves, I., Finetti-Sialer, M. M., Hirsch, P. R., Ward, E., Devonshire, J., & Hidalgo-Díaz, L. (2013). Pochonia chlamydosporia: Advances and Challenges to Improve Its Performance as a Biological Control Agent of Sedentary Endo-parasitic Nematodes. Journal of Nematology, 45(1), 7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625126/
Mendoza, G. A. ., Wilson, J. H. ., & Colina, J. C. (2013). Efecto de Trichoderma atroviride, Trichoderma harzianum y Trichoderma viride sobre huevos de Meloidogyne sp. en condiciones de laboratorio. Revista Científica de Estudiantes Rebiolest, 1(2), e65. https://revistas.unitru.edu.pe/index.php/ECCBB/article/view/479
Morton, C. O., Hirsch, P. R., Peberdy, J. P., & Kerry, B. R. (2003). Cloning of and genetic variation in protease VCP1 from the nematophagous fungus Pochonia chlamydosporia. Mycological Research, 107(1), 38–46. https://doi.org/10.1017/S0953756202007050
Puertas, A., De la Noval Pons, B. M., Martinez, B., Miranda, I., Felix, F., & Hidalgo-Diaz, L. (2006). Interacción de Pochonia chlamydosporia var. catenulata con Rhizobium sp., Trichoderma harzianum y Glomus clarum en el control de Meloidogyne incognita. Revista de Protección Vegetal, 21(2), 80–89. https://agris.fao.org/agris-search/search.do?recordID=CU2009100416
Puertas, A., & Hidalgo Díaz, L. (2009). Efecto de diferentes abonos orgánicos sobre el establecimiento de Pochonia chlamydosporia var.catenulata en el sustrato y la rizosfera de plantas de tomate. Revista de Protección Vegetal, 24(3), 165. http://revistas.censa.edu.cu/index.php/RPV/article/view/457
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Jose M. Alomia-Lucero, Eliseo Capcha-Ospina

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors retain their rights:
a. The authors retain their trademark and patent rights, as well as any process or procedure described in the article.
b. The authors retain the right to share, copy, distribute, execute and publicly communicate the article published in the Revista Agrotecnológica Amazónica (RAA) (for example, place it in an institutional repository or publish it in a book), with an acknowledgment of its initial publication in the RAA.
c. Authors retain the right to make a subsequent publication of their work, to use the article or any part of it (for example: a compilation of their works, notes for conferences, thesis, or for a book), provided that they indicate the source of publication (authors of the work, journal, volume, number and date).









